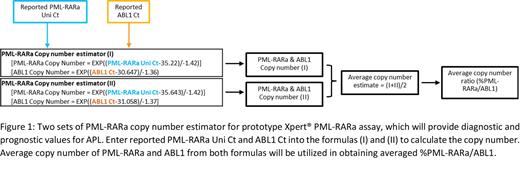
Statement of the Problem: Acute Promyelocytic Leukemia (APL) represents 10-15% of Acute Myeloid Leukemia (AML). The PML-RARa fusion transcript is expressed in more than 95% of APL patients. Three PML-RARa isoforms (bcr1, bcr2, and bcr3) are identified in 90-95% of PML-RARa positive cases. Prototype Xpert ® PML-RARa, an automated cartridge-based assay for measuring PML-RARa fusion transcript levels (bcr1, bcr2, and bcr3), is standardized to quantify the amount of PML-RARA relative to ABL1 control gene based on delta Ct in peripheral blood. Since PML-RARa level is crucial for diagnosis and ongoing therapeutic monitoring in APL, it can be useful to obtain the PML-RARa copy number (CN). The aim of this work is to develop PML-RARa CN estimator and to compare %PML-RARa/ABL1 reporting between delta Ct-based and CN-based methods. Methodology & Theoretical Orientation: Four levels of PML-RARa (bcr1, bcr2, and bcr3) and ABL1 IVT-RNA panels as well as two lots of prototype Xpert ® PML-RARa assay were used to generate standard curves for CN and %CN reporting. The samples with spiked-in bcr1 IVT-RNA and APL clinical samples containing PML-RARa fusion transcript were examined to evaluate the CN and %CN between two lots of the prototype Xpert ® PML-RARa assay and to compare the delta Ct-based and CN-based methods for reporting %PML-RARa/ABL1. Findings: Linearity was demonstrated in Ct vs CN input for PML-RARa (R 2>0.98) and ABL1 (R 2>0.97). Less than 2-fold difference was exhibited for CN and %CN across two different lots. Less than 2-fold difference was observed in %PML-RARa/ABL1 reporting between delta Ct-based and CN-based approaches. Conclusion & Significance: A PML-RARa copy number estimator for prototype Xpert® PML-RARa assay was established, which will provide helpful information for diagnosis and prognosis of APL. (Product in development. Not for use in diagnostic procedures. Not reviewed by any regulatory body.)
Disclosures
Yuan:Cepheid: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal